6th Gene Therapy Analytical Development & CMC Summit 2024
We covered the full range of analytical methods across bioassays, molecular biology, physicochemical properties, process development and CMC, our comprehensive 3-track agenda provided the technical detail and knowledge you needed to:
Understand how to transition your potency assay from early to late-stage
Explore how NGS methods can better inform your genome titers
Accurately measure partials in your samples
Produce contaminant-free and high yield vector batches at scale
Leverage automation and in-process analytical tools to improve CMC efficiency on a platform level
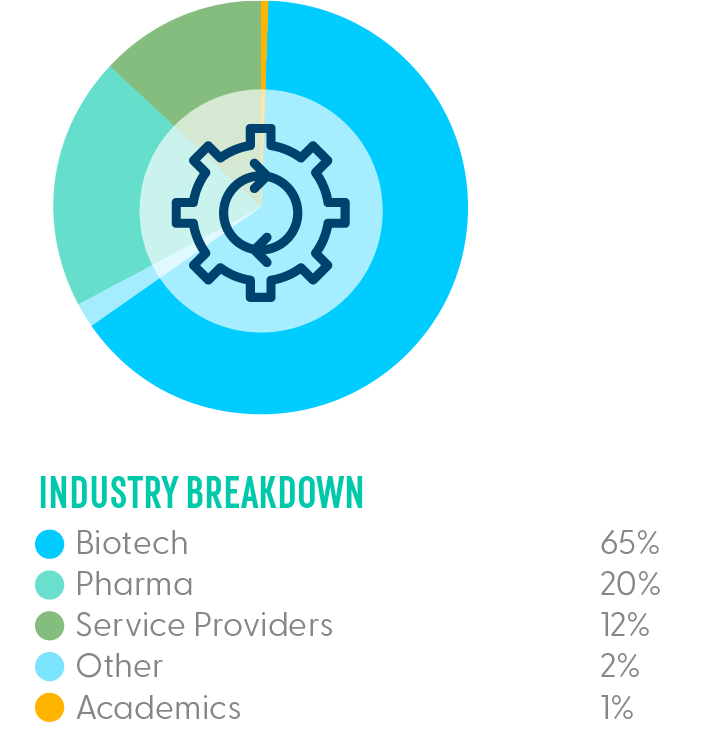
Companies from biotech, pharma, and key service providers shared data-driven and case-study-led technical presentations on new technology and modifications to existing analytical processes for AAV-based gene therapies. They also designed analytics for novel gene therapy capsids beyond traditional AAV and gene transfer to provide comprehensive, in-depth, and up-to-date information on the state of play in 2024.
What Was New for 2024?
Leverage Brand-New Data & Case Studies to Better Characterize Your Gene Therapy Products
Optimize your Full CMC Package Strategy to Meet Regulatory Requirements
Unparalleled Networking Opportunities with Analytical & CMC Experts in Gene Therapy
Understand how the Latest Analytical Innovations can be Applied in Practice
Improve Upstream & Downstream Process Development to Ensure Product Quality & Reduce Cost of Goods
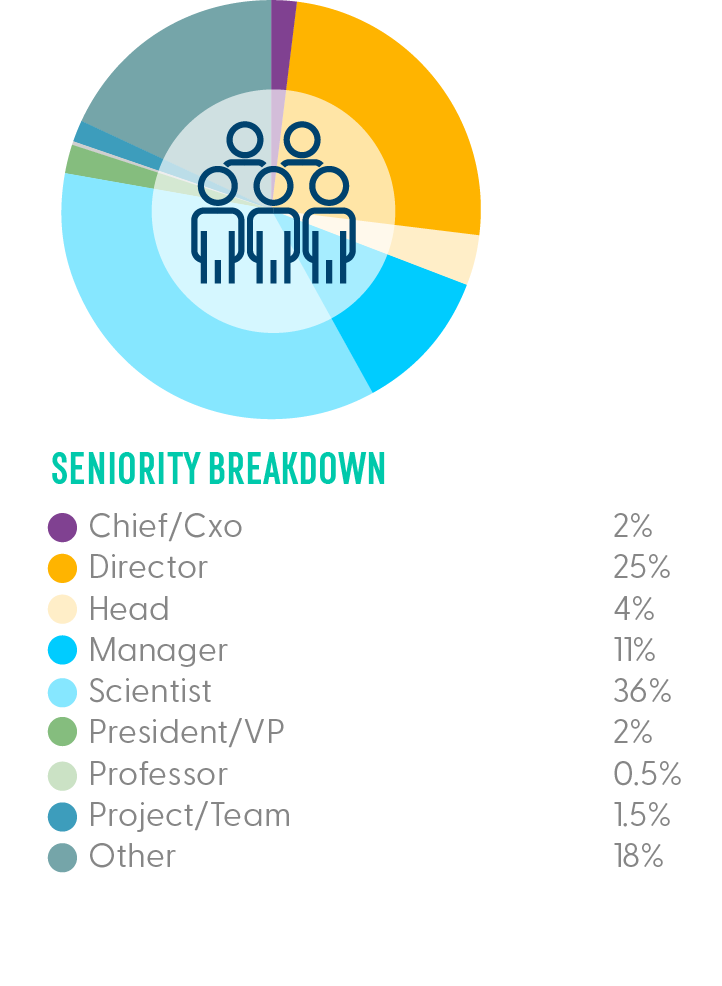
Who Was in the Room?
We joined teams across analytical development, assay development, analytical chemistry and process development from the leading biotech and pharma companies in the space sharing technical and data-driven content on analytics for gene therapies across molecular biology, bioassays, physicochemical properties and CMC to cover the breadth and depth of analytical methods.